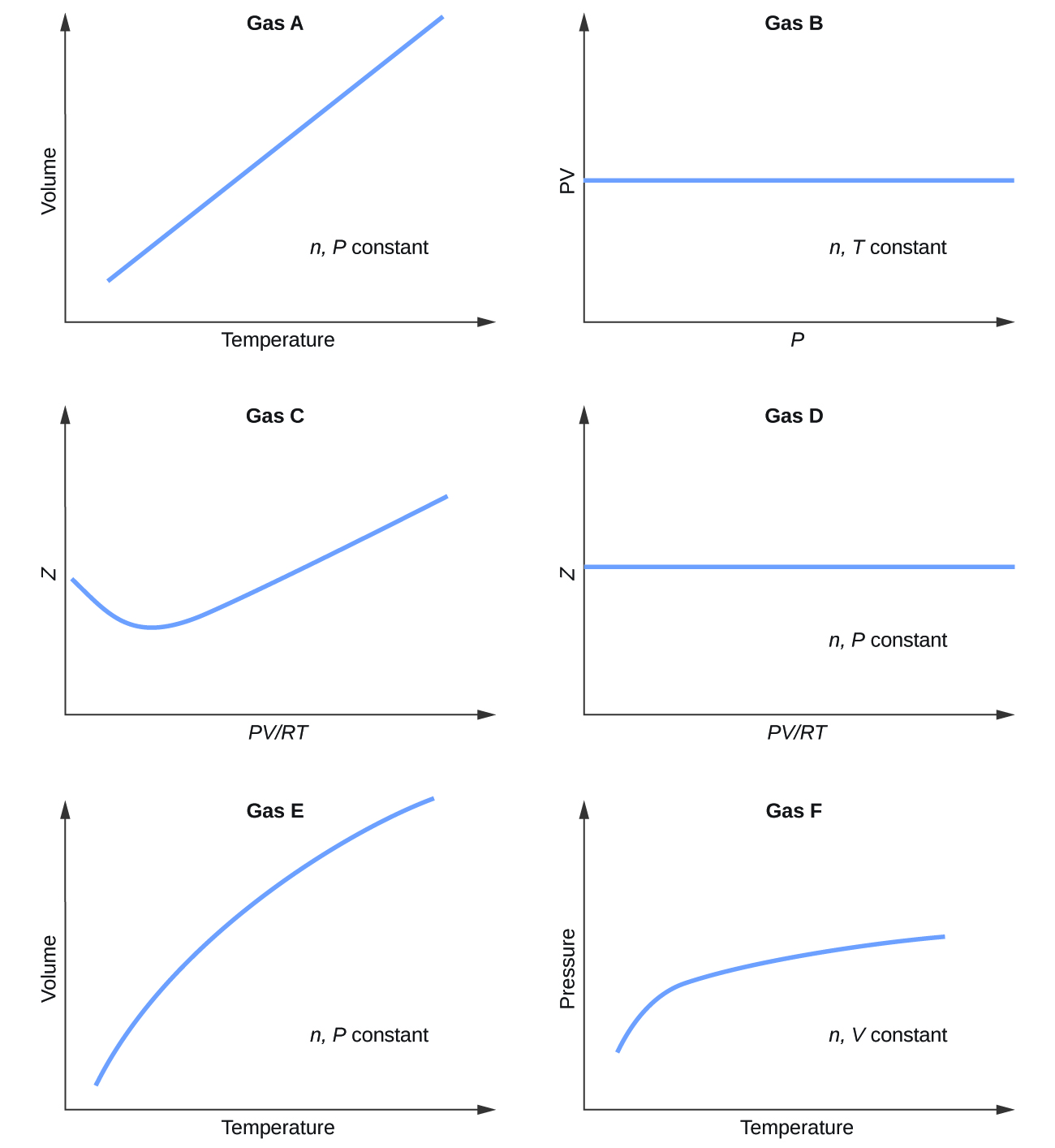
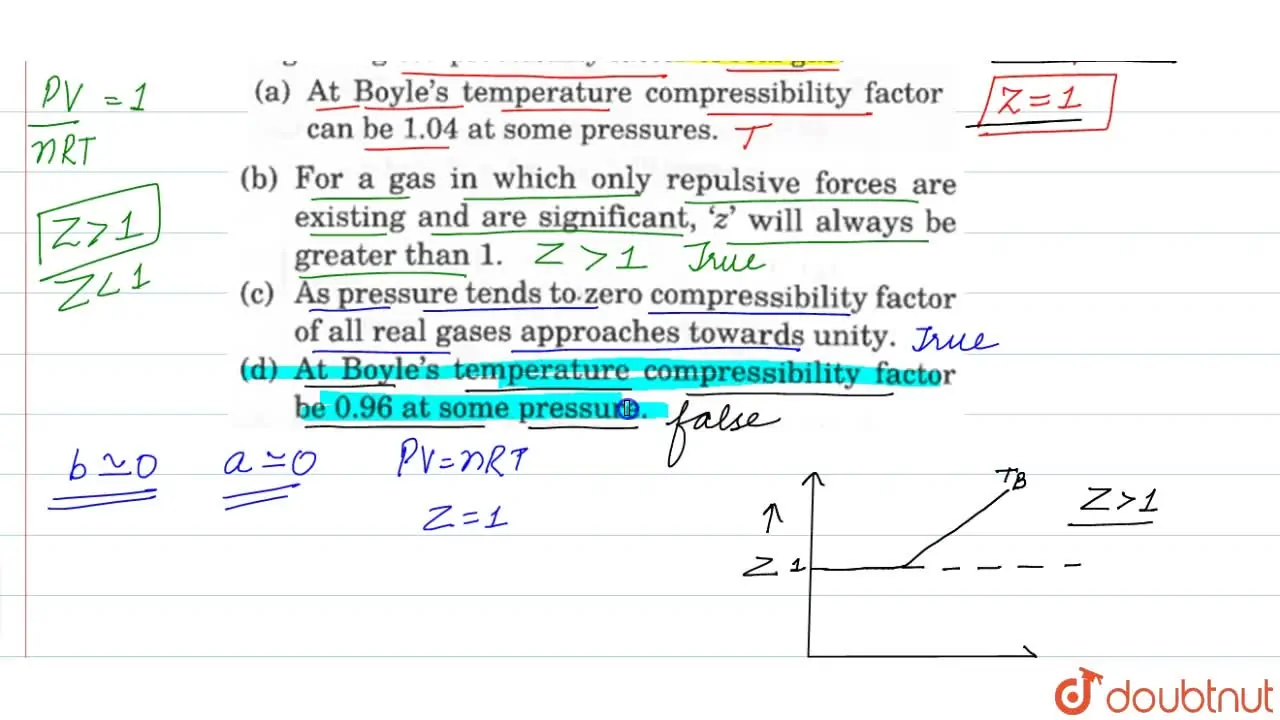
Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for
$ 25.99 · 4.8 (706) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases
Analysis of some factors affecting the water vapour diffusion in soils

Non-Ideal Gas Behavior Chemistry: Atoms First
8.6 Non-Ideal Gas Behavior – General Chemistry 1 & 2
/app/uploads/sites/28/201
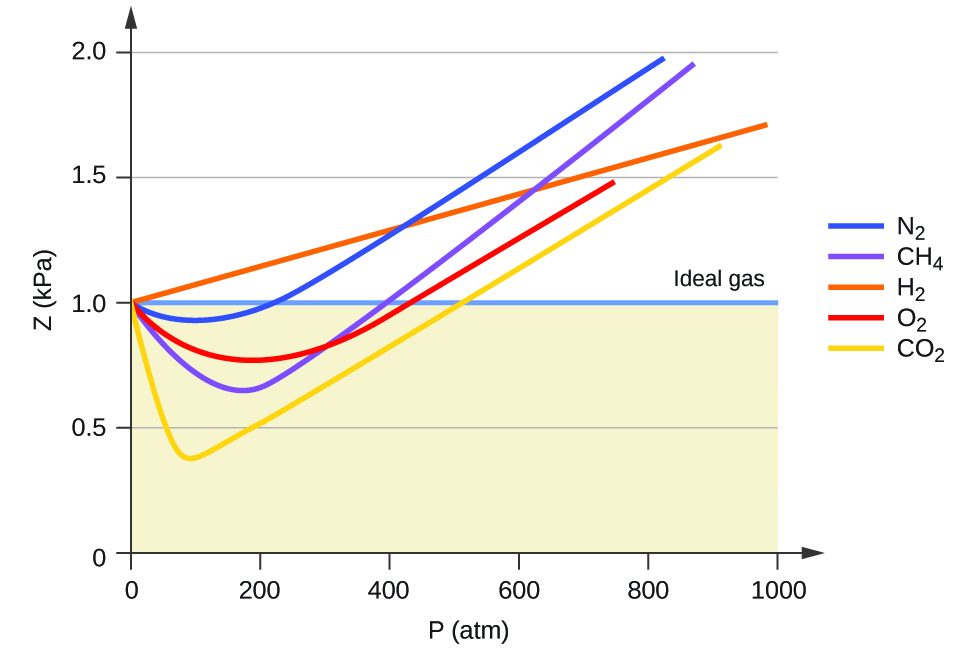
As pressure tends to zero compressibility factor of all real gases app

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p

Which of the following statement is wrong ? For gas A, a= 0 and z will linearly depend on pressureFor gas B, b = 0 and z will linearly depend on pressureGas

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Yucation The given graph represent the variations of Z (compressibility factor = pV) v/s p three nRT real gases, A, B and C. Identify the incorrect statement. p(atm) - A. For the

The given graph represents the variation of Z(compressibility factor =- PV nRT ) versus P, three real gases A, B and C. Identify the only incorrect statement. Ideal gas P (atm) (A)

Solved True or False? The compressibility factor of any gas

Which of the following statements is wrong according to the given graph?