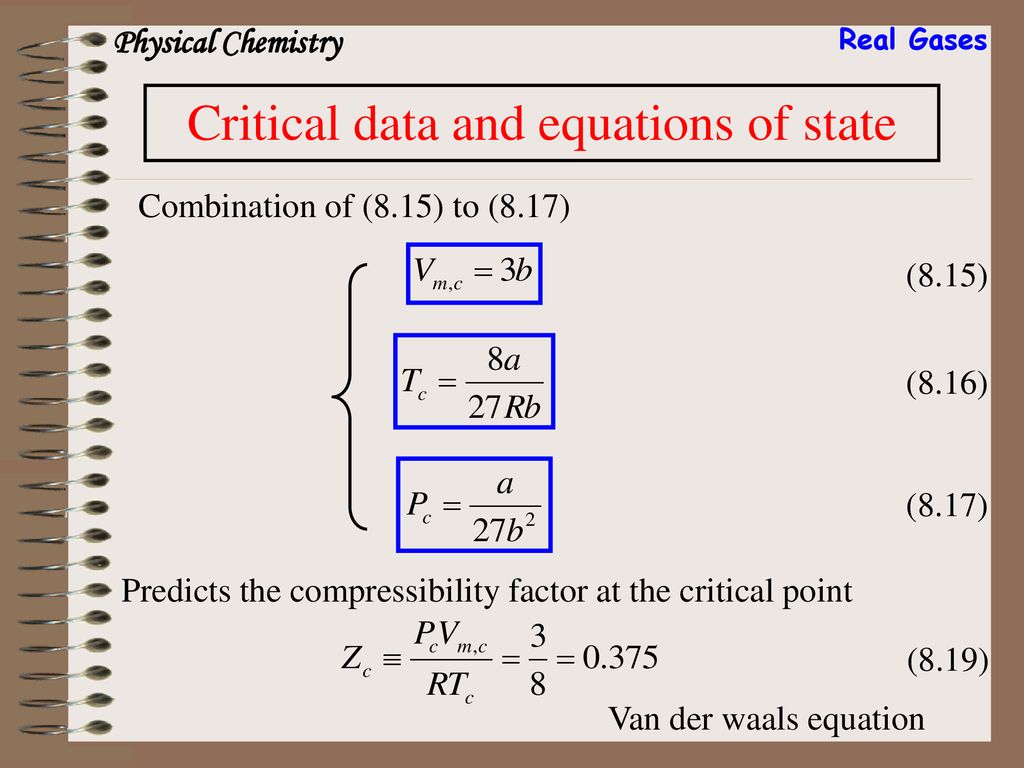
Solved The virial expansion of the compression factor (Z)
$ 20.99 · 4.9 (236) · In stock
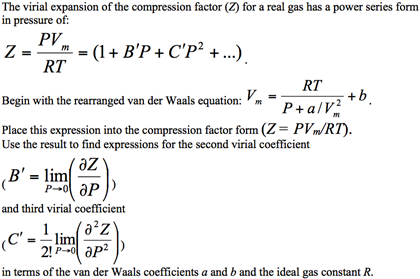
Solved Consider a Virial expansion equation P=RTP+ RTP+(BY

SOLVED: The virial expansion of the compression factor (Z) for a real gas has a power series form in pressure of: PV = 1 + BP + CP + RT. Begin

Virial Expansion Providing of the Linearity for a Unit

Physical Chemistry The Compression Factor (Z) [w/1 example

Virial Expansion Providing of the Linearity for a Unit

The Compression Factor, Z, and Real Gases - What you NEED to Know

Show that (equation) where alpha is the expansion coefficient and
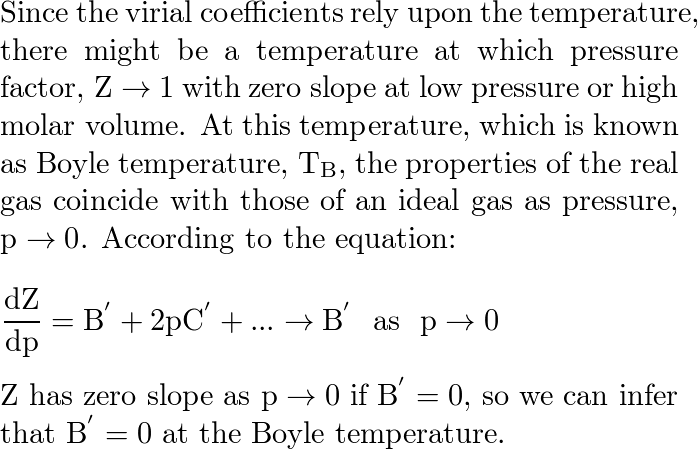
The second virial coefficient of methane an be approximated
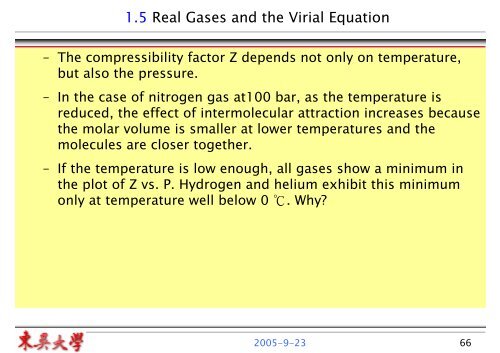
1.5 Real Gases and the Virial Equation - Mail

Solved The compression factor is given by Z = pV/RT = 1 +
86. Prove that Z at high pressure for 1 mol is 1+b/V b

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry
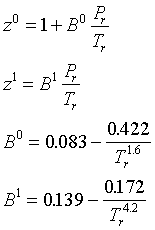
Thermodynamic Models