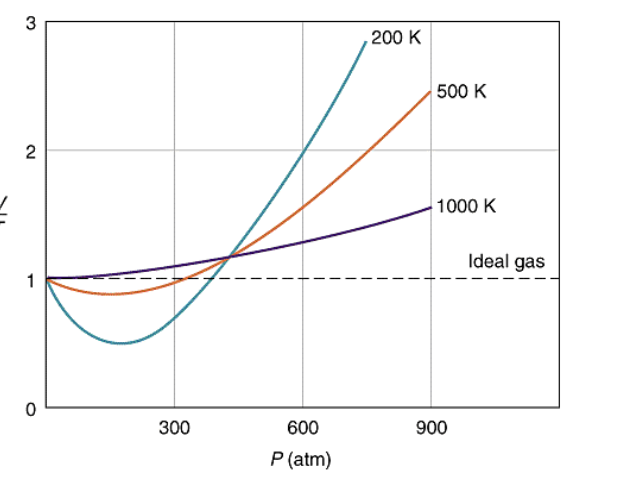
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
$ 22.50 · 4.5 (360) · In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
Solved 1. The plot below shows how compressibility factor

Consider the graph between compressibility factor Z and pressure P
Why does gas liquefy at high pressure? Even at high-pressure

Production Engineering - ScienceDirect

PDF) Field Operational Problems due to Condensate Formation in

PDF) Field Operational Problems due to Condensate Formation in Retrograde Gas Reservoirs
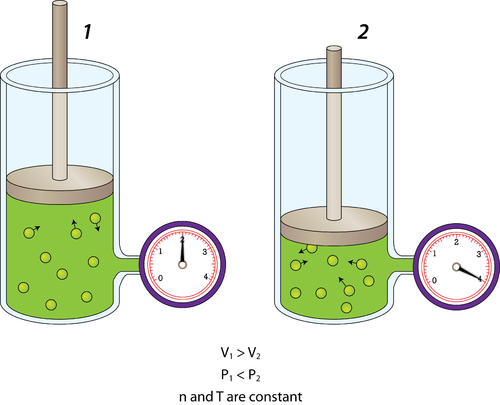
The Behavior of Gases Chemistry for Non-Majors

gaseous state

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `P` for three real gases `A`, `B`, and `C`. Identify the incorrect - Sarthaks eConnect