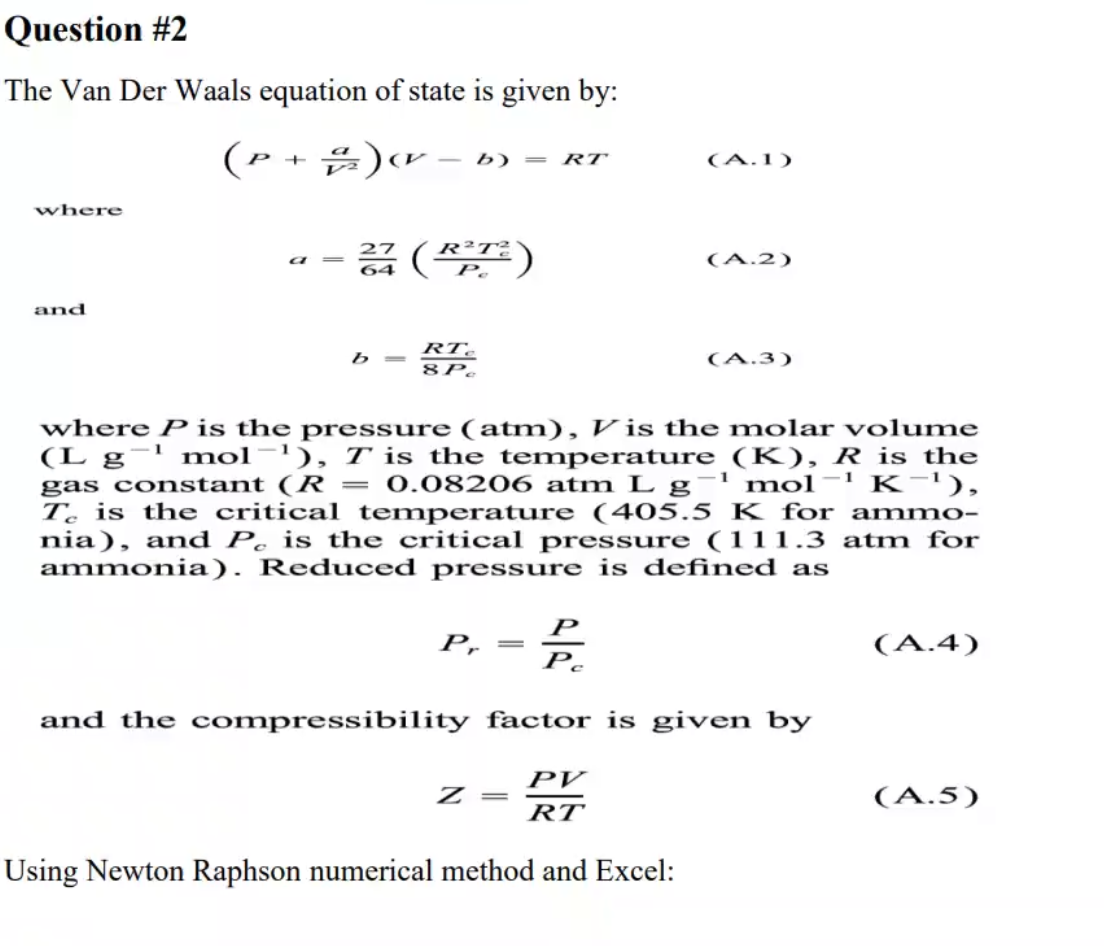
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
$ 9.50 · 4.7 (250) · In stock
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as
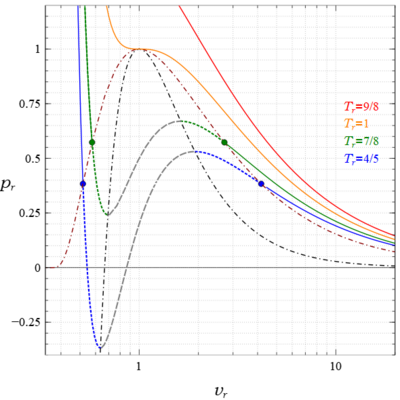
Van der Waals equation - Wikipedia

At a high pressure, the compressibility factor (Z) of a real gas is us
Solved 2. (20 points) At low pressures, the compressibility

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

Solved APPENDIX Problem 1: Molar Volume and Compressibility

⏩SOLVED:For a van der Waals gas with given values of a and b,…
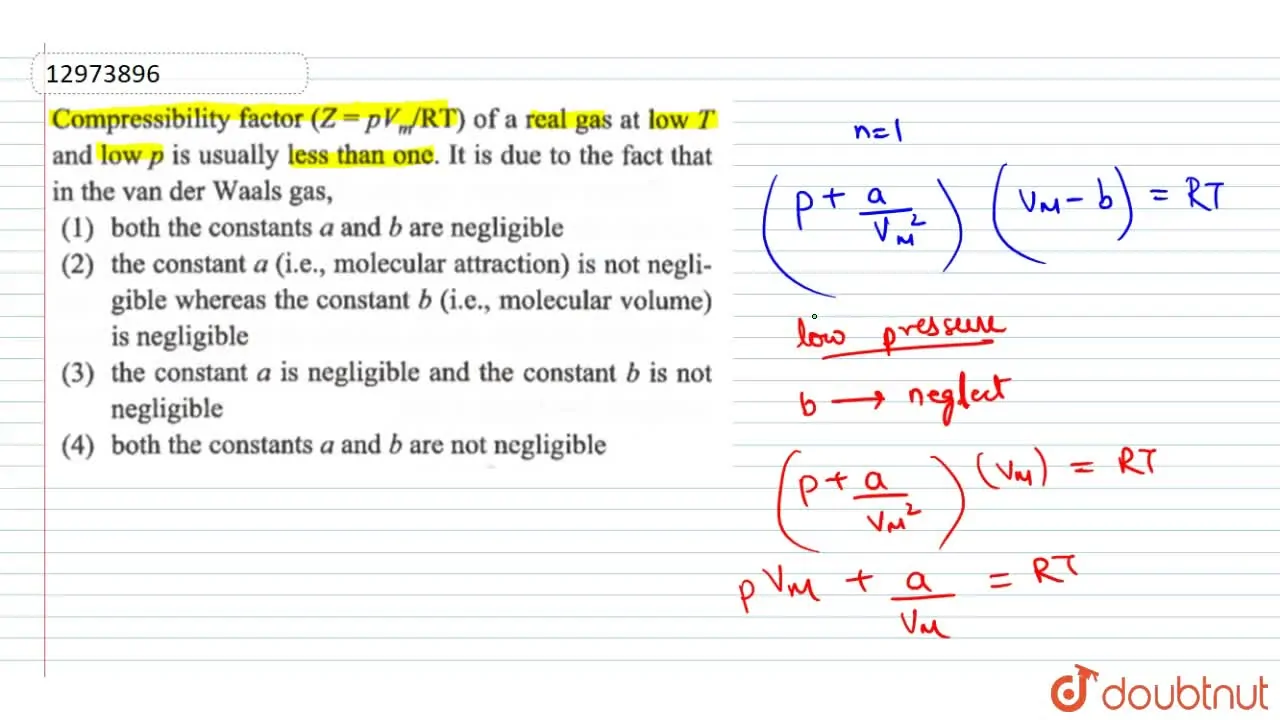
the constant a is negligible and the constant b is not negligible

If Z is a compressibility factor, van der Waal's equation low pressure can be written as : tot gnolaszemit sem st263 nisho ad Phim shuplamenu Pb (1) Z = 1 - (

Van der Waals equation - Wikipedia

Compressibility Factor Calculator - File Exchange - MATLAB Central
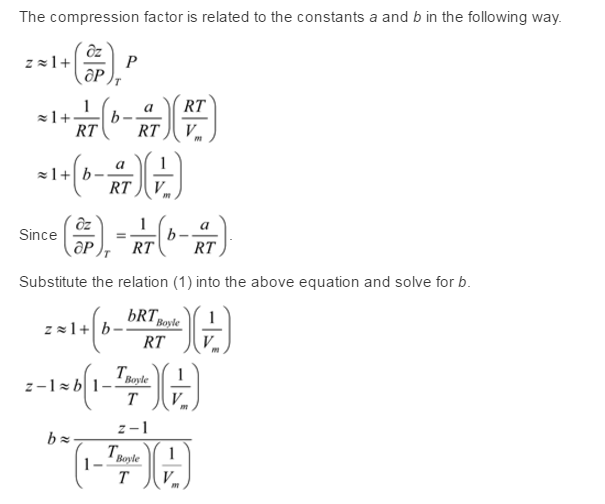
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
Solved The Van Der Waals equation of state is given by

Compressibility factor (gases) - Citizendium
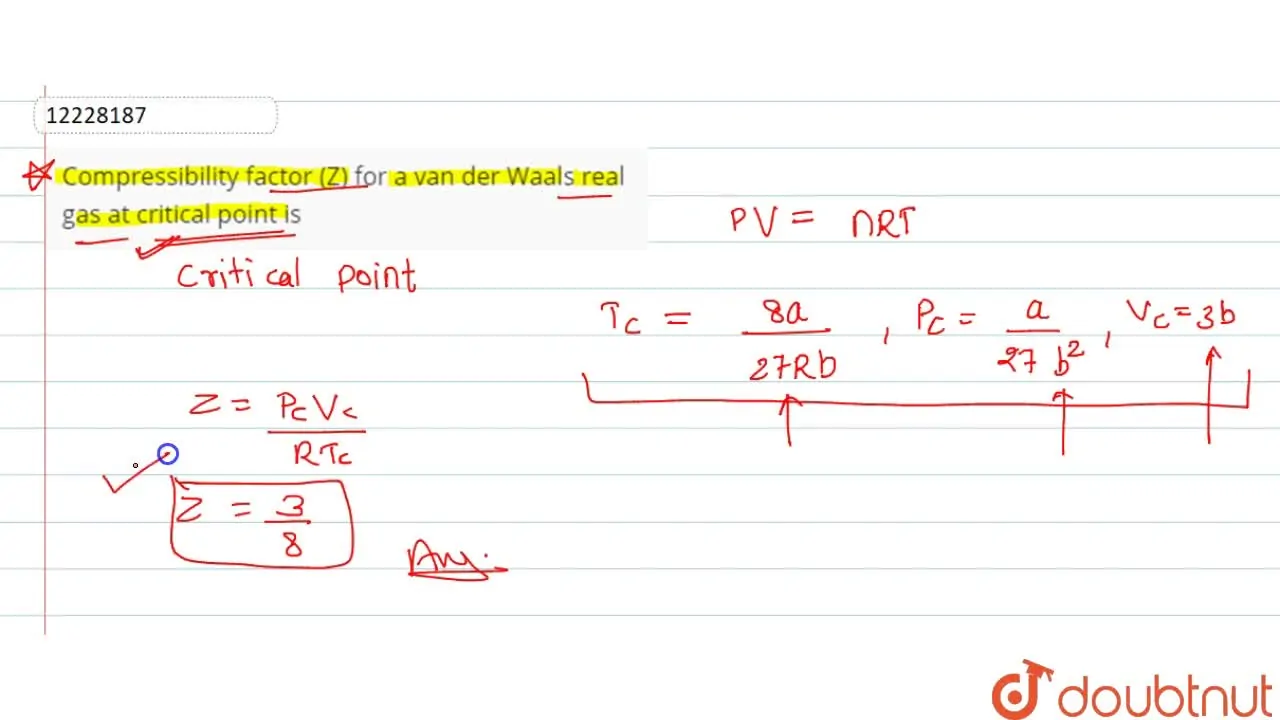
Compressibility factor (Z) for a van der Waals real gas at critical po

Solved Problem 2. ( 30 points) The ideal gas law can