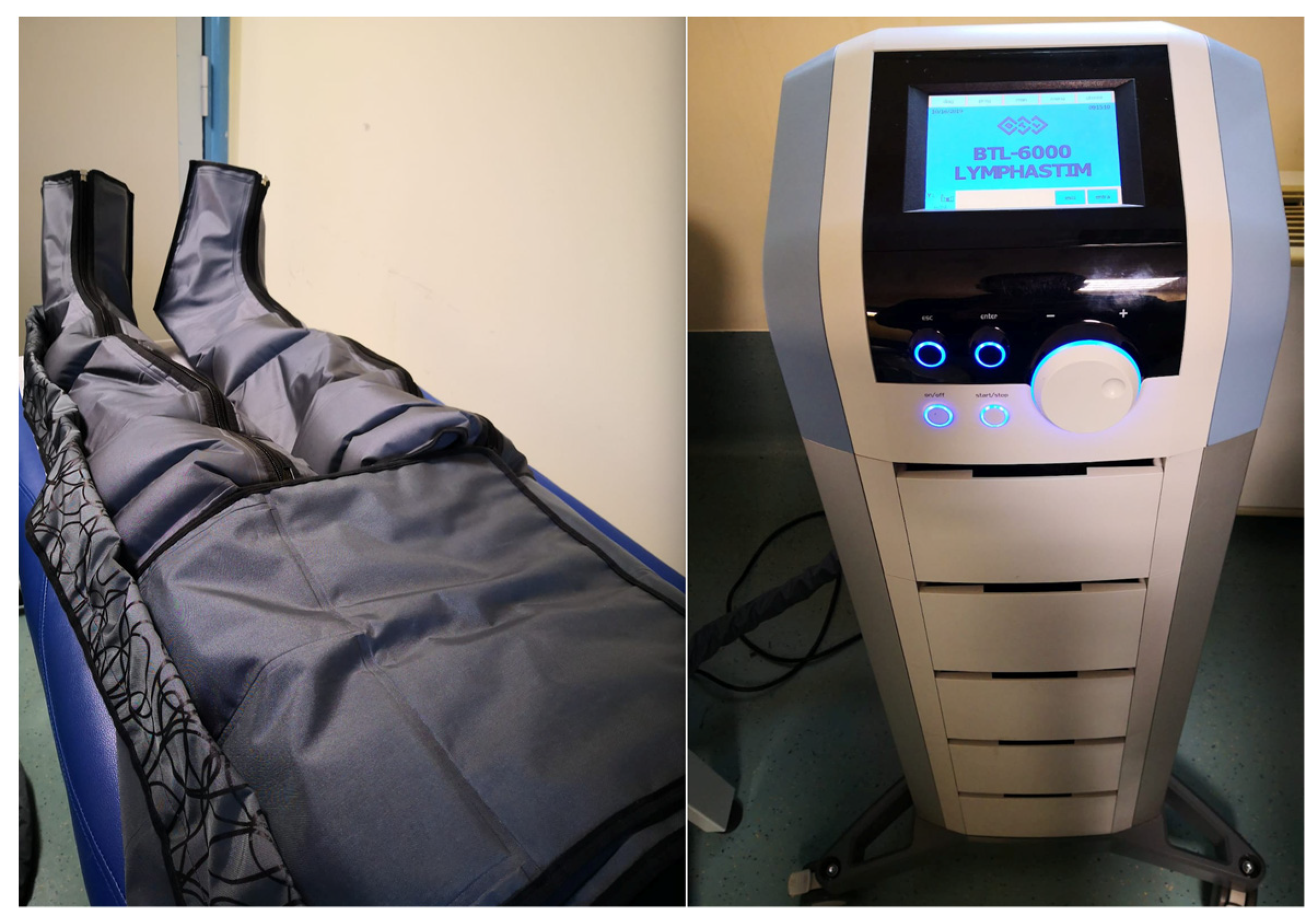
AIROS Medical Receives FDA Clearance to Market New Peristaltic
$ 21.99 · 4.9 (768) · In stock

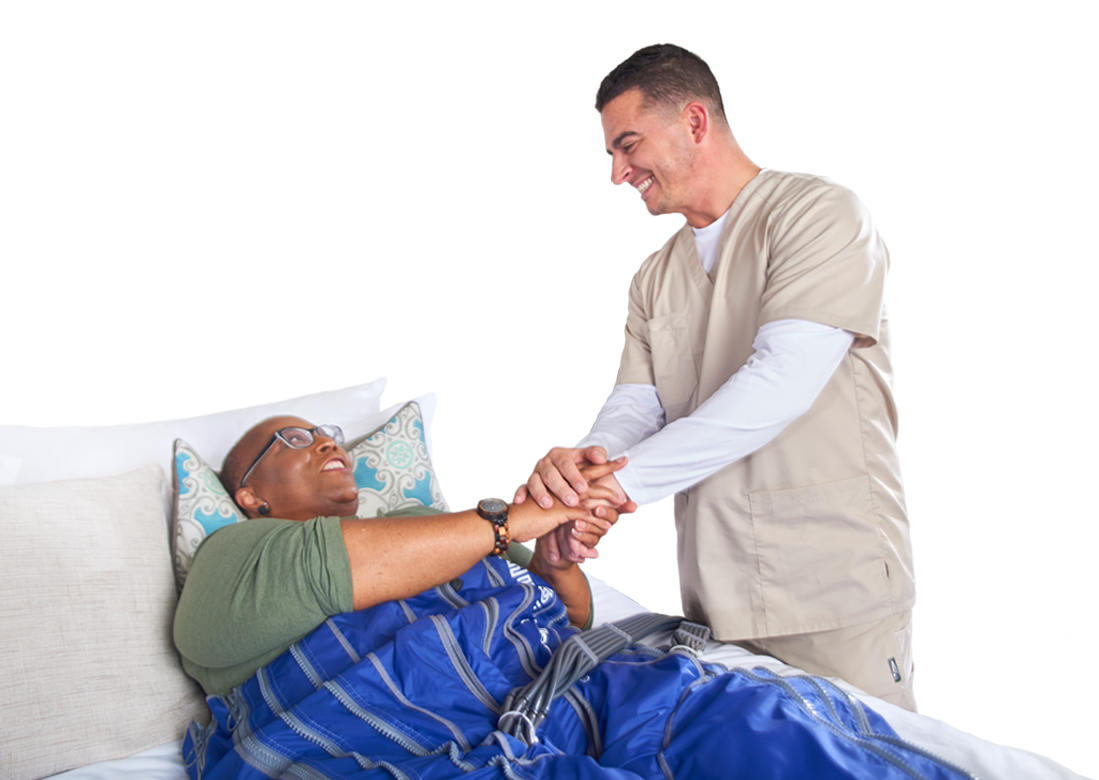
AIROS Medical announces FDA 510k clearance to market the AIROS 8P compression device and Pants garment that treats leg and pelvic swelling.

FDA approves Beyond Air LungFit PH to treat hypoxic respiratory failure

AIROS Medical Launches Sales of Compression Therapy Devices and

Airway Clearance System Market – Global Industry Trends and

Darren Behuniak, Author at AIROS Medical, Inc.
AIROS Medical Receives FDA Clearance to Market New Peristaltic

Press Room Archives - AIROS Medical, Inc.

FDA Approved EO Gas Sterilizers
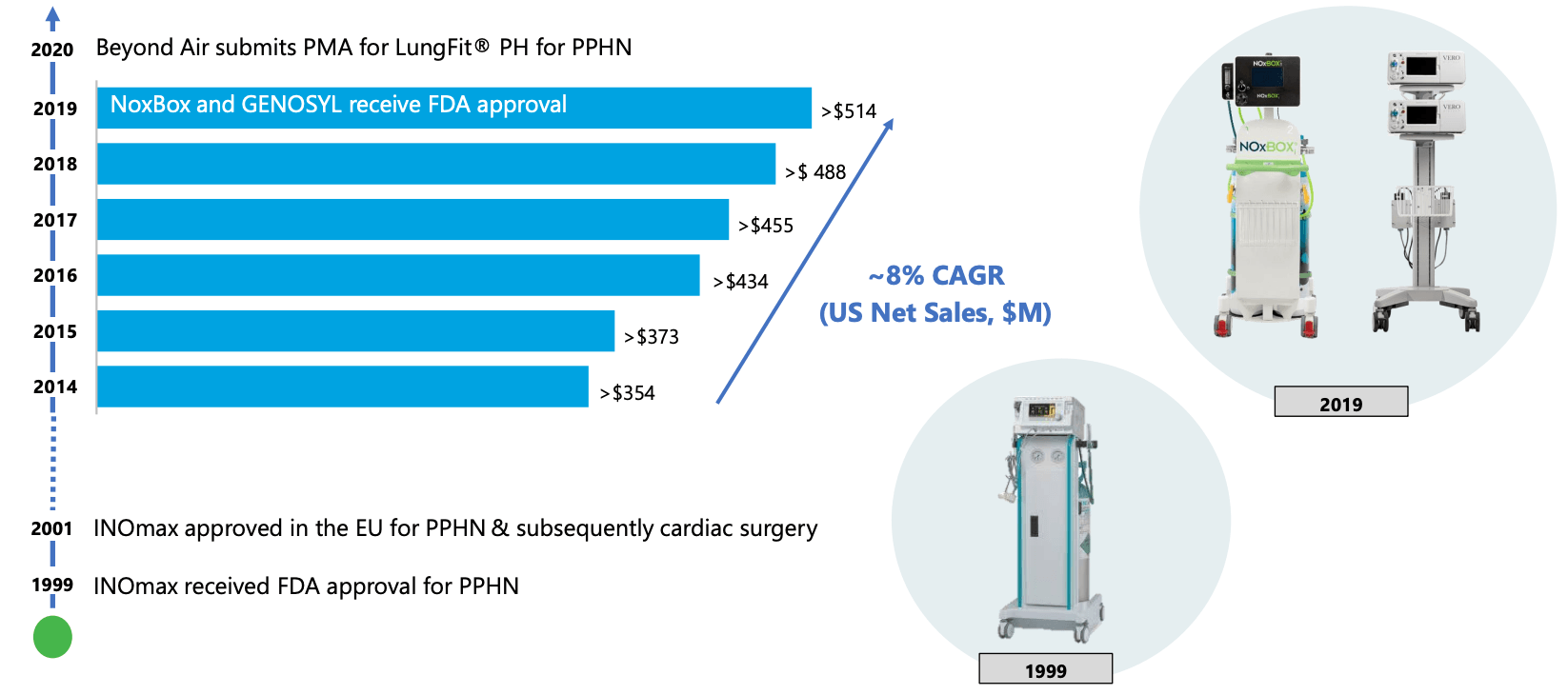
Beyond Air Stock: Tiny Company With Truly Massive Potential (NASDAQ:XAIR)

AIROS Medical Granted FDA 510k Clearance to Market Compression Device for Lymphedema Treatment
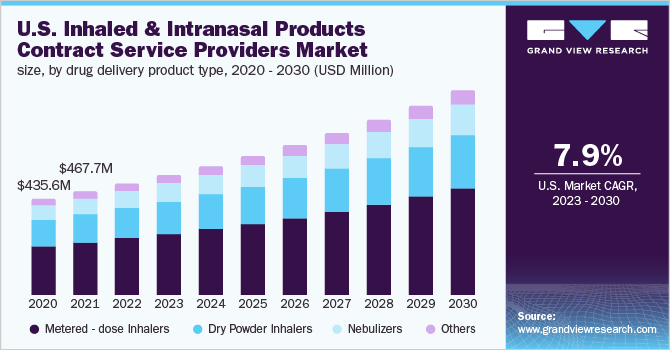
Inhaled And Intranasal Products Contract Service Providers Market Report, 2030

Compression Wear And Shapewear Market Trend Analysis, And Forecast To 2033

FDA investigates urological endoscopes - Andersen Sterilizers

VWR® Traceable® 2-Channel Thermometer