How to Calculate Normality of a Solution
$ 15.00 · 4.7 (528) · In stock
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
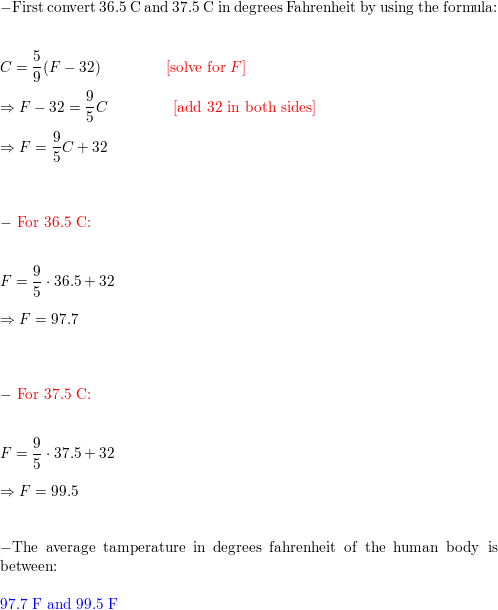
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.
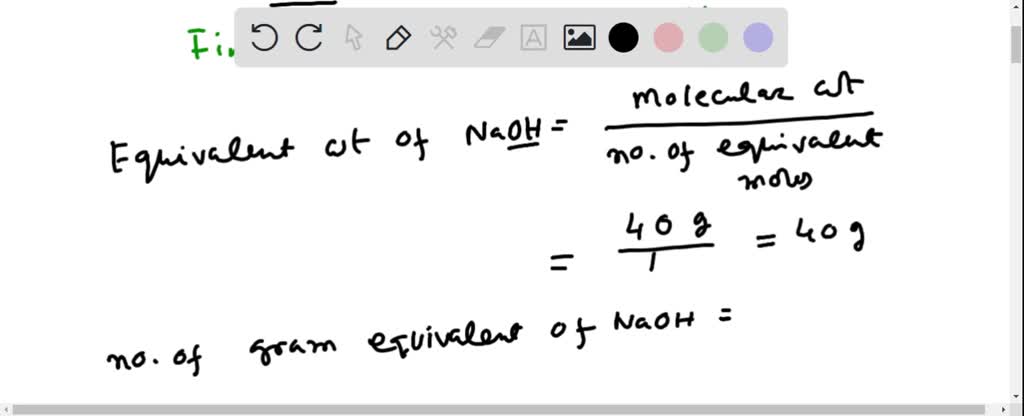
SOLVED: Calculate the normality of NaOH solution formed by dissolving 0.2 gm NaOH to make 250 ml solution: Select one: 2.0N 2.5N 0.2N 18N

Bengali] Calculate the normality of a solution containing 3.15g of hy
Lesson 7: Acids and Bases

SOLUTION: Difference between molarity molality and normality and how to calculate them - Studypool
What is the normality of 1.2M H2SO4? - Quora
Solved] This is a Normality Problem that already shows the solution. I am

Calculate the volume strength of H_(2)O_(2) solution if 50 mL of H_(2)O_(2) solution is diluted
Determine: Show all calculations 1. Normality (N) of
What is the molarity of 0.4 normality of H2SO4 solution? - Quora

The normality of solution containing 31.5g of hydrated oxalic acid (H₂C₂O₄.2H₂O) - NEETLab
What is the normality of a 15% HNO3 solution with a density of 1.12g/mL? - Quora
Lesson 7: Acids and Bases