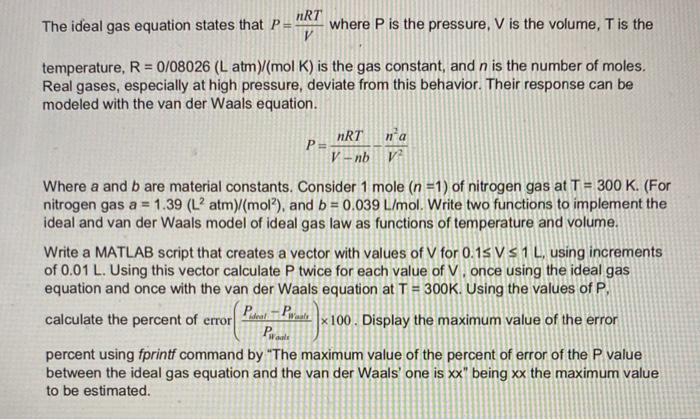
Solved) - NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, (1 Answer)
$ 7.99 · 4.6 (313) · In stock
amp;#160;NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, N Is The V Number Of Moles Of Gas, R= .08206, T Is The Temperature (In Degrees Kelvin), And V Is The Volume Of The Gas. At High Pressure, A More Accurate Equation Is The Van NRT

How to Calculate Partial Pressure: Step-by-Step Solution

Energies, Free Full-Text
Solved nRT The ideal gas equation states that P= V where P
Kannada] Derive the relation between Density and Molar mass of a gase
How to prove ideal gas law(pv=RT) - Quora
Processes, Free Full-Text
Understanding the Fundamental Gas Laws: The Ideal Gas Equation
What is the ideal gas law? - Quora

What is n in the equation of the ideal gas law?
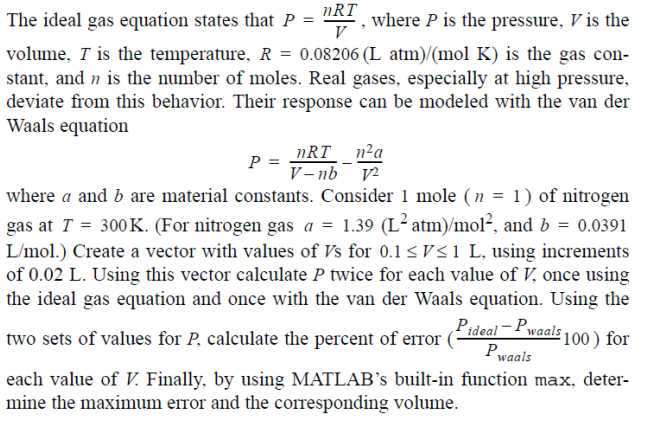
Solved nRT The ideal gas equation states that P = , where P
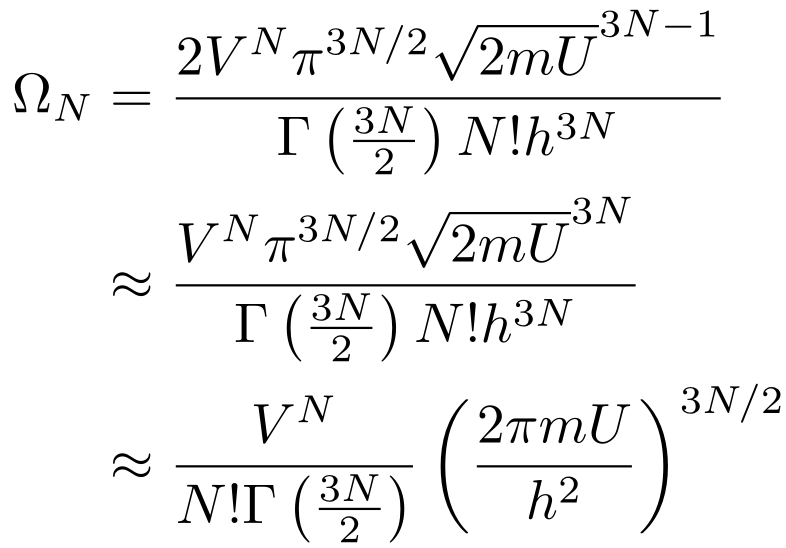
Let's Derive the Ideal Gas Law from Scratch!







/i.s3.glbimg.com/v1/AUTH_bc8228b6673f488aa253bbcb03c80ec5/internal_photos/bs/2023/f/9/rJMAlPQ3yROozAJ3cTSg/mg-3450.jpg)