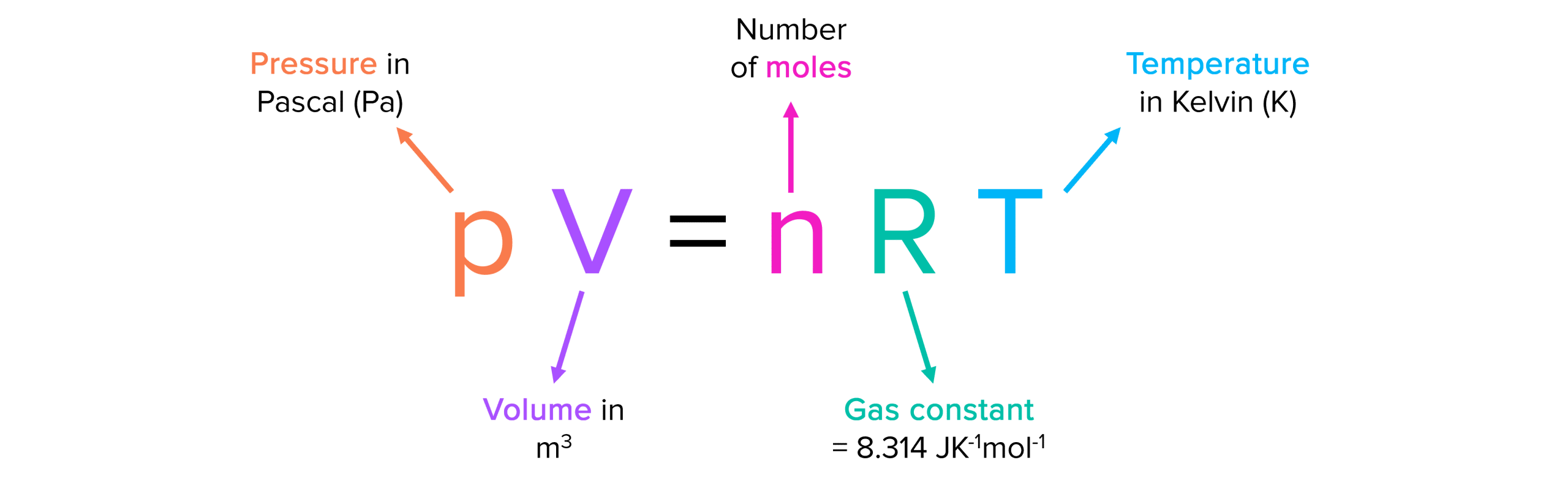10.4: The Ideal Gas Equation - Chemistry LibreTexts
$ 27.00 · 4.7 (461) · In stock

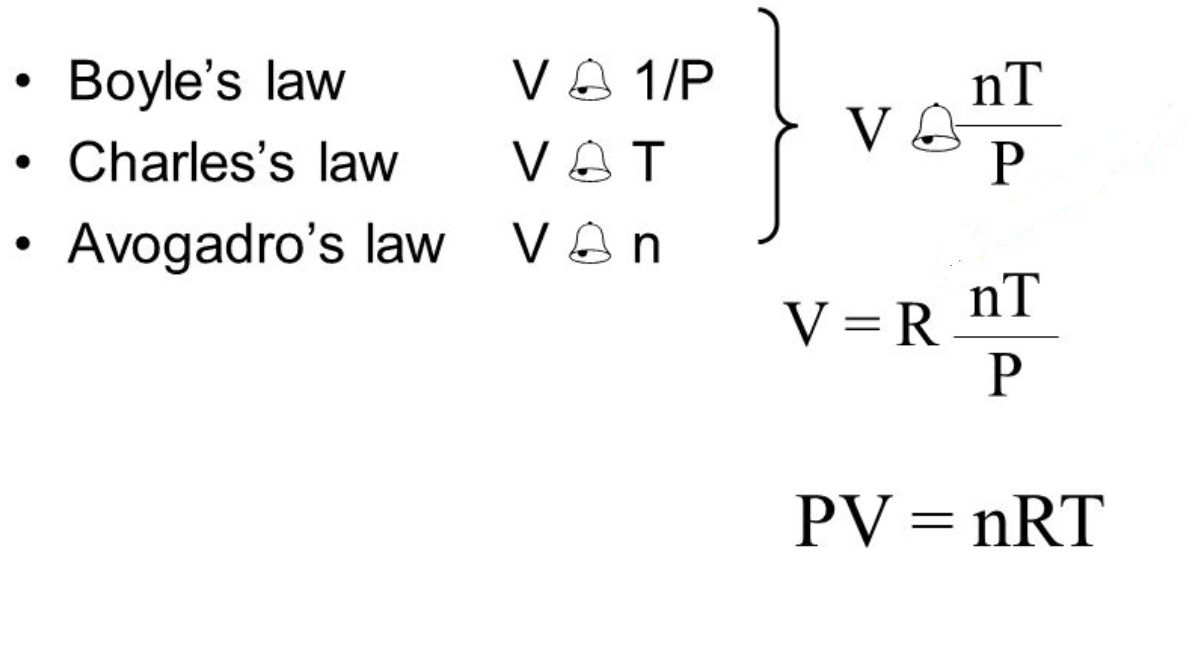
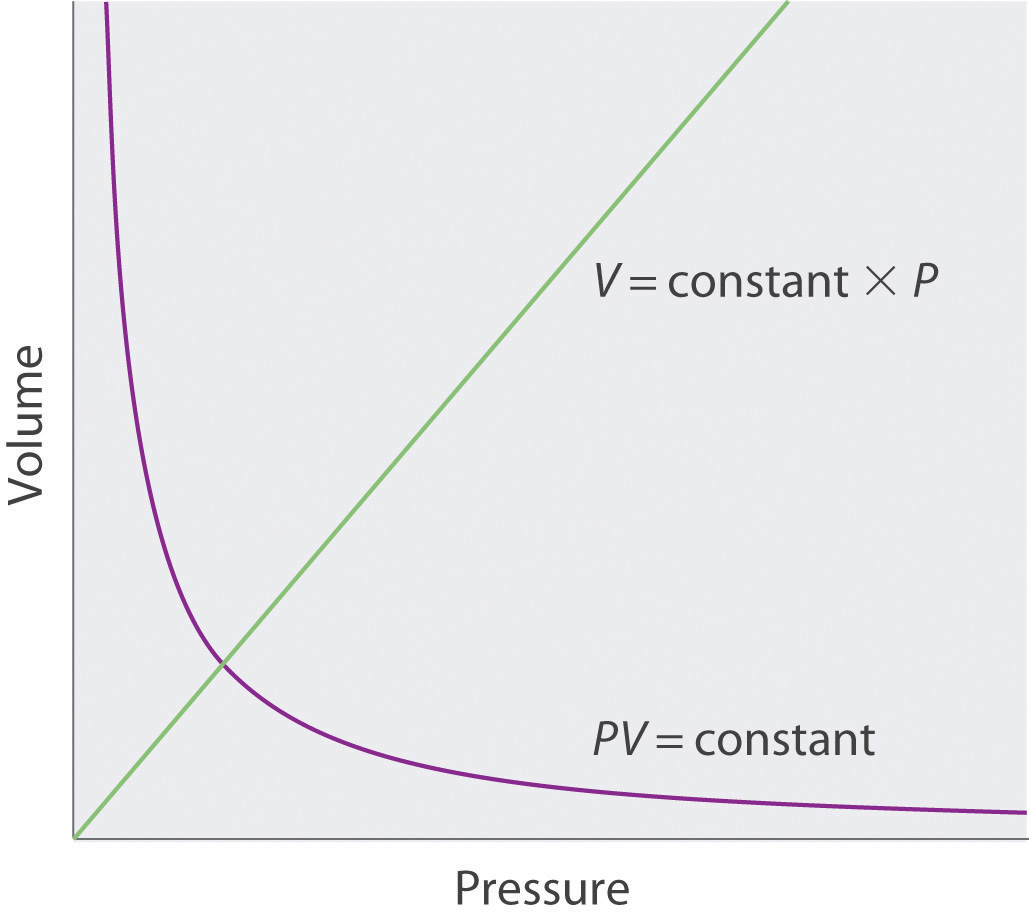
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the …
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the gas constant. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Standard temperature and pressure (STP) is 0°C and 1 atm.
Chapter 10.4: The Combined Gas Law - Chemistry LibreTexts
The Ideal Gas Law Boundless Chemistry

Chemistry - 2e - Open Textbook Library
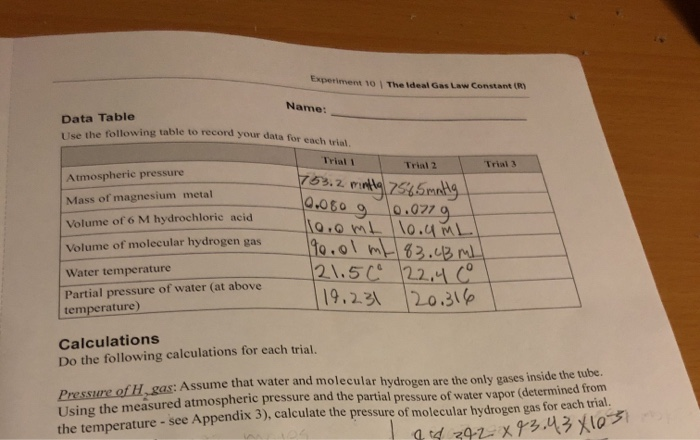
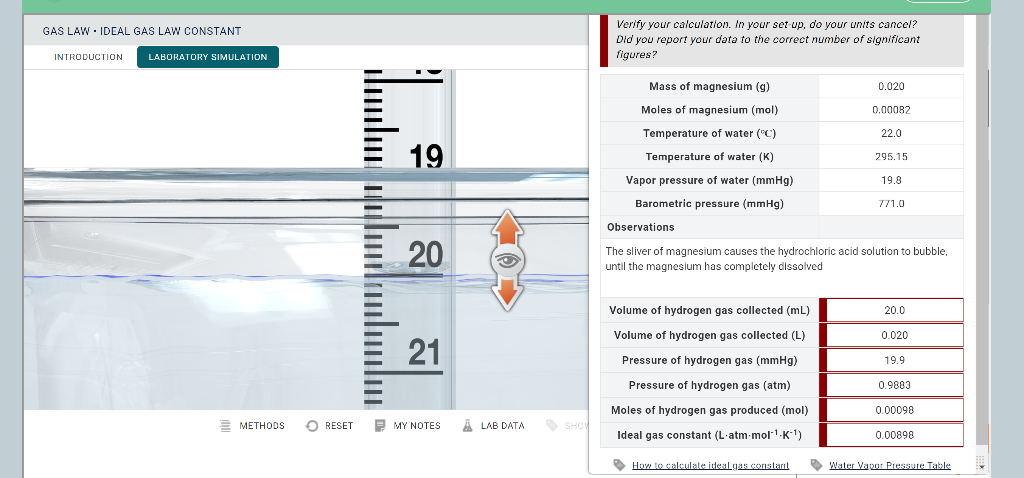
Solved Experiment 10 The ideal Gas Law Constant (R) Name

Ionization energy
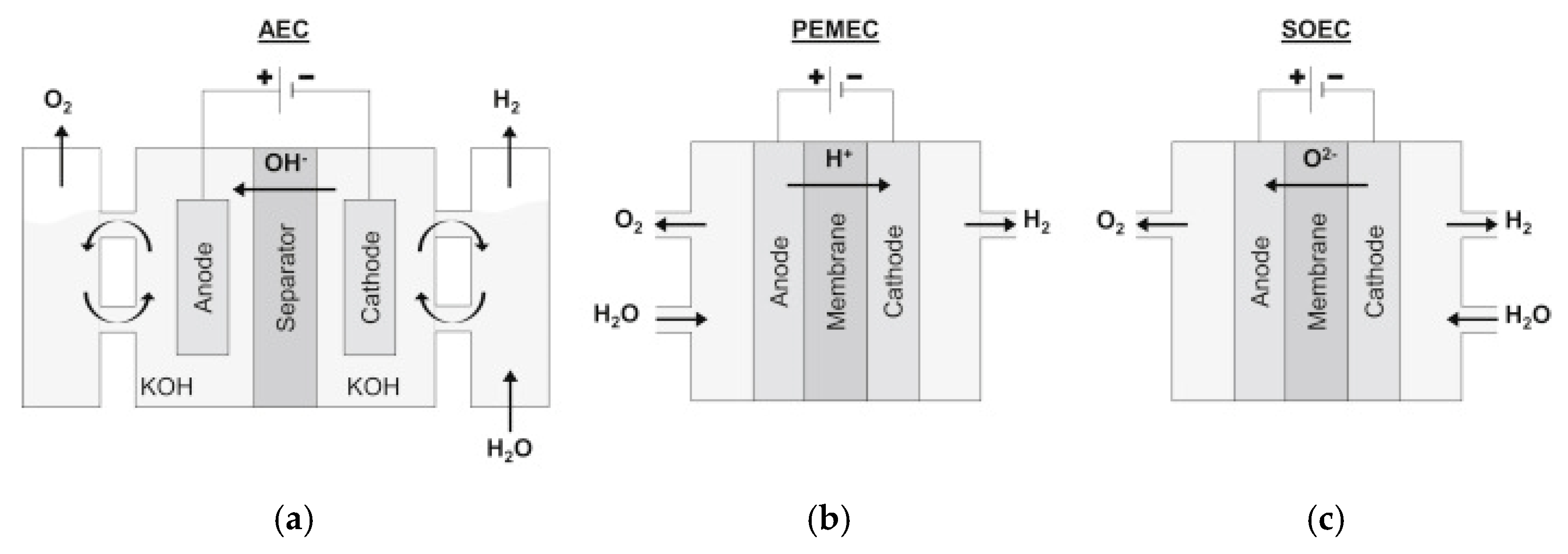
Energies, Free Full-Text
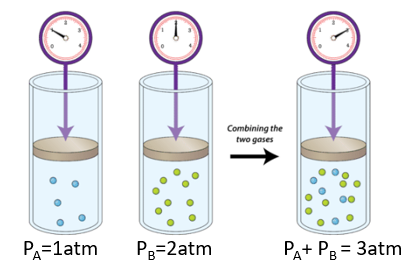
10.4: Gas Mixtures - Chemistry LibreTexts

Chapter 10 Gases. - ppt video online download
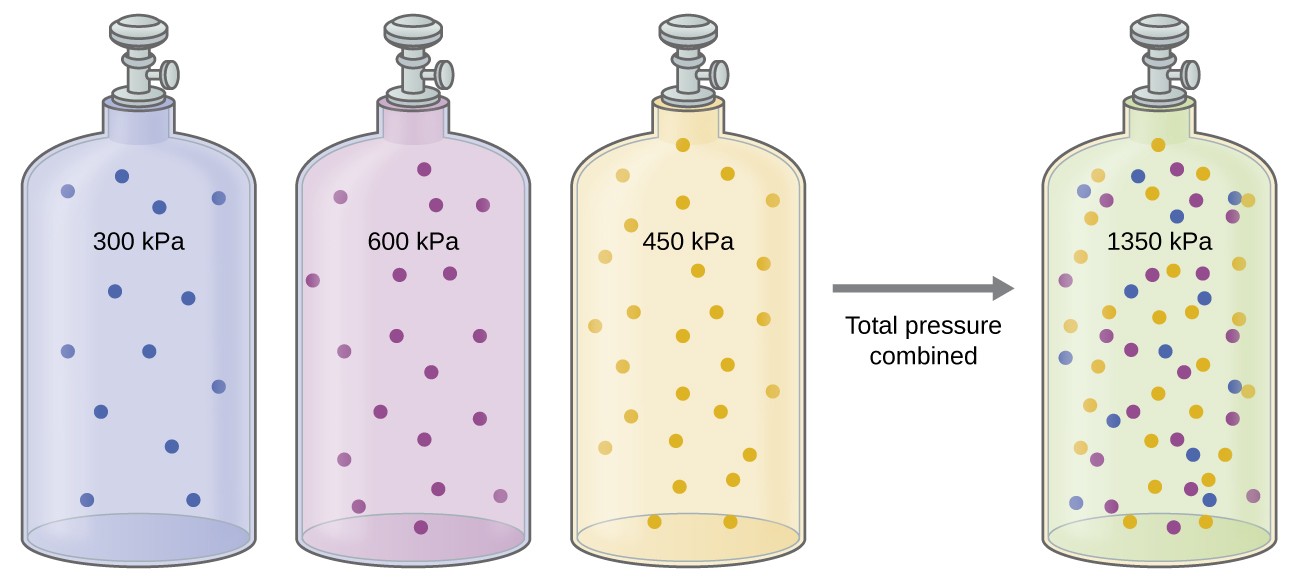
10.4: Stoichiometry of Gaseous Substances, Mixtures, and Reactions - Chemistry LibreTexts

4.1: The Perfect Gas - Chemistry LibreTexts
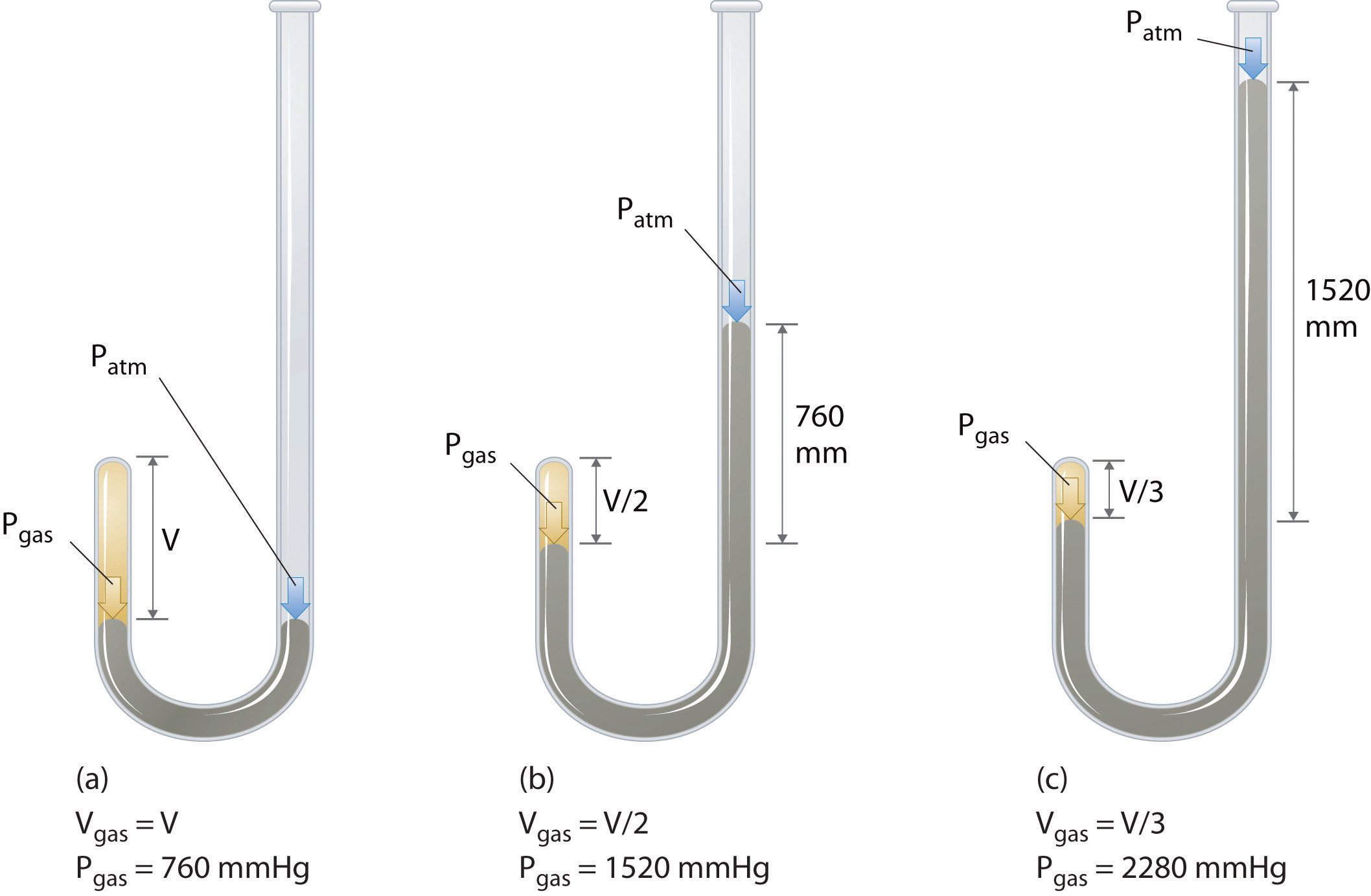
Chapter 10 Gas Laws
Solved GAS LAW IDEAL GAS LAW CONSTANT Aleks I worked on this
10.E: Exercises - Chemistry LibreTexts