What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
$ 14.50 · 4.7 (700) · In stock


02 mole of a van der Waals gas pressure of 0.1 alin. Civanges unpredictably (B-16. What is the compressibility factor (Z) 0.02 mole of a Assume the size of gas molecules is

Elasticity, Strength, and Water Permeability of Bilayers that Contain Raft Microdomain-Forming Lipids: Biophysical Journal

Solved We begin by showing that the compressibility factor
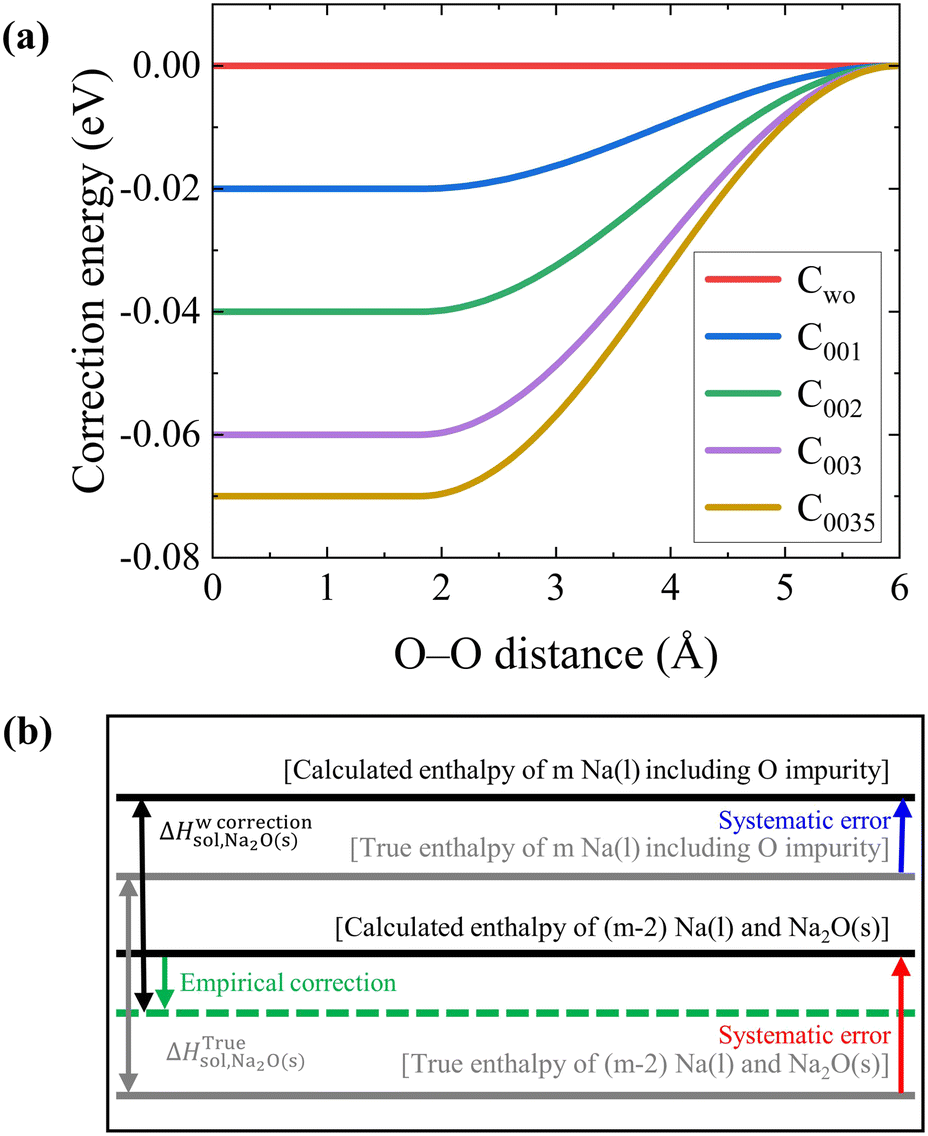
Temperature dependence of O solubility in liquid Na by atomistic simulation of Na(l)–Na 2 O(s) interfaces using corrected machine learning potential: - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D3CP01348K
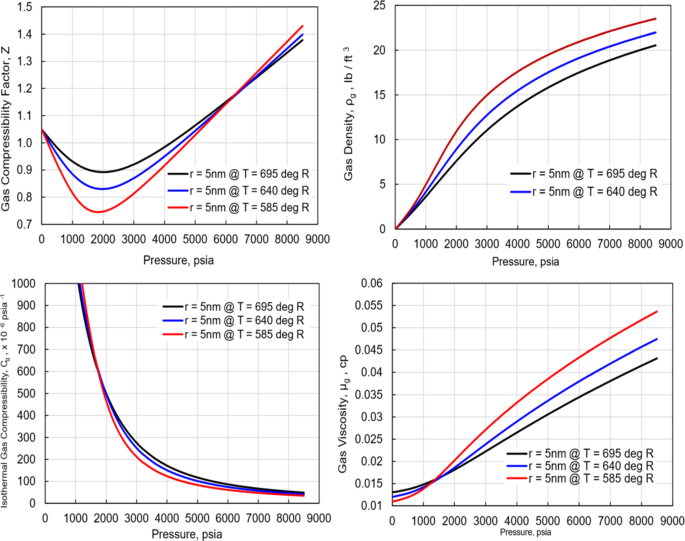
Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift

Van Der Waals Equation - an overview

Van Der Waals Equation - an overview
Real Gases, PDF, Gases
Real Gases, PDF, Gases
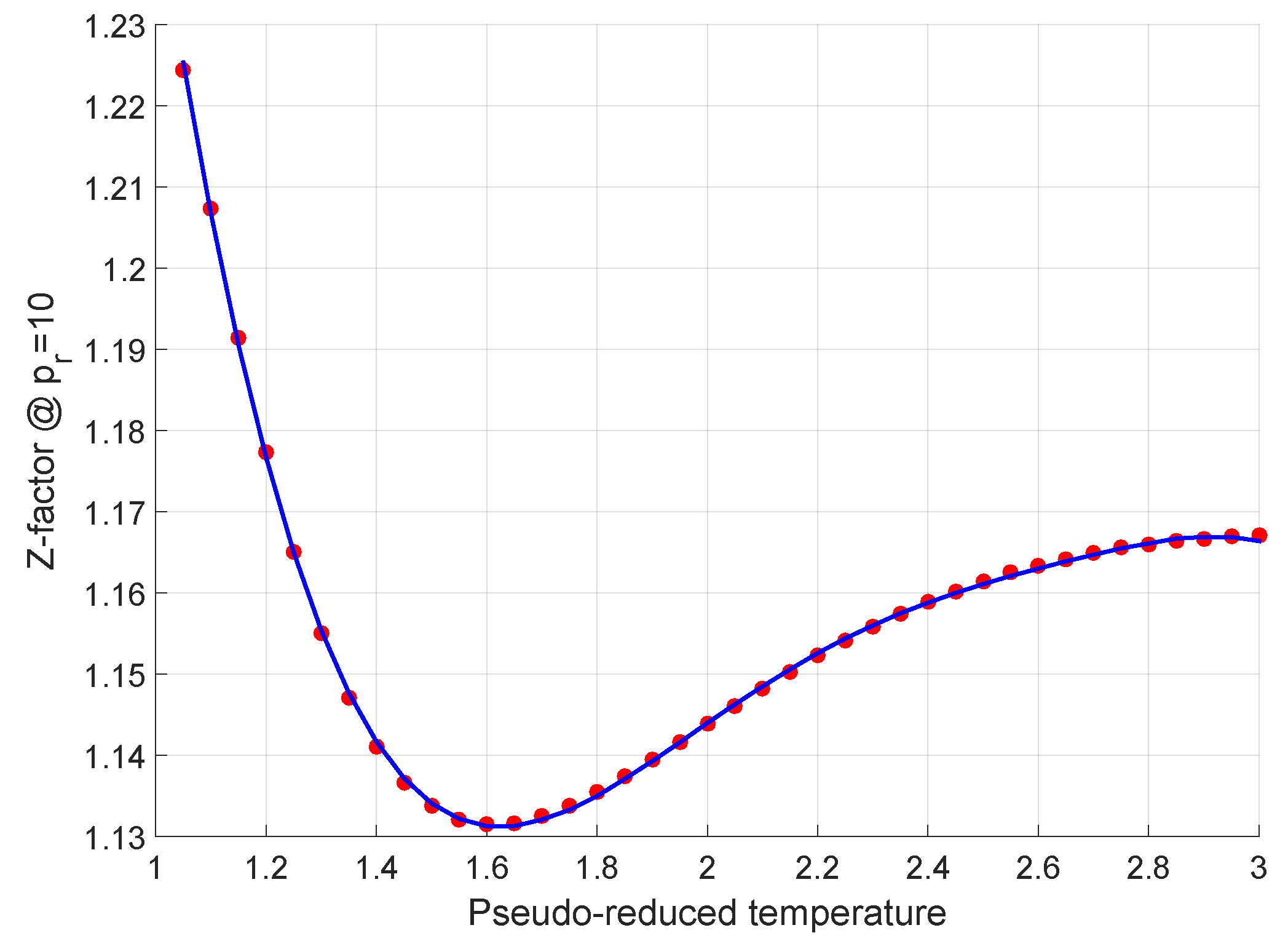
Energies, Free Full-Text

McMurry and Fay On-Line Chapters
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift

63. What is the compressibility factor (2) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given : RT = 20









